Most people assume that using disposable cups is universally bad for the environment. In truth, it depends on how they are disposed of and what else would be used in the place of disposable cups. In this post, I focus solely on the energy necessary to create each type of cup, to clean them, and how many re-uses are needed for a reusable cup to have a per-use energy cost lower than a disposable cup. The lifecycle energy use for disposable cups vs. ceramic and plastic cups have a surprising result: a restaurant will need to use a cup nearly 300 times to have break-even energy use*. Home use can be much better**, thanks to our much more lax health standards at home.
The scope of this post is limited to energy, which fails to provide a whole picture view. The next post will discuss other aspects, including pollution and greenhouse gases made during production, disposal, the intrinsic value of having a recycling mindset, and details about how the differing sources of energy used for production make a huge impact on these other factors.
The first part of this post will be pretty boring for most of you, containing basic explanation of units and dimensions that you will already know. If this is old hat to you, skip past the “basic background” section. It is meant for people who are unfamiliar with the basic physics behind energy who might be rusty calculating energy requirements for heating something.
If you know basic physics, skip down to the part beginning with "lifecycle energy assessment"
Basic Background – Energy, Joules, Heat, and Power
The really basic stuff: heat (most people should skip to next section, unless you know zero physics)
This section briefly goes over the basic units of energy. The unit of energy is a joule. It takes 4.2 joules per gram of water to raise it one degree Celcius (or about 2 degrees Fahrenheit). This number, 4.2 is called the specific heat of water.
This brings up another important concept: heat vs temperature. Everyone knows what temperature is. Not everyone knows what heat is. Heat is a measure of how much energy you have to dump into one gram of a substance to raise it one degree. Water, as we said, takes 4.2 joules. Iron take about one tenth of that, or .45 joules. Think about it this way: if you have a quarter of cup of nearly boiling 90°C water, and it falls on you, you are going to the hospital for some burn treatment. If you have a similar mass of 90°C iron fall on you, the heat of it won’t do much damage (although if it falls from high enough, it might hurt). So, roughly, for a given temperature, something with a higher specific heat has a lot more energy.
How much heat energy does it take to heat a large amount of water? To do this, we take the mass, multiply by the specific heat (represented by c), and then multiply by temperature change: m*c* ΔT, where the Δ means change, and Δt means change in temperature.
Less basic stuff
A cup of water is about 250 milliliters, or 250 grams. Raising a cup of coffee to 40° Celsius (about 100° F) from 10° Celsius would take:
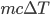
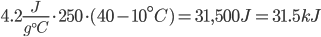
Notice that the units of degrees Celsius and grams cancel out, with 1/(gC) from specific heat being multiplied by g and C from the weight and temperature parts of the equation, leaving only Joules. Sweet.
Anyways, 31,500 J is also written as 31.5 kJ (31.5x103 J). Let’s take a moment to convert this to kwh, or kilowatt hour, the standard unit of energy you pay for in your house. A watt is using one joule per second. If we took the coffee cup above, and wanted to heat it in 30 seconds, we would expend 1050 watts for 30 seconds (31.5kJ/30s). How many kwh is this? 31,500J/3600s = 8.75 watt-hours, or .00875 kwh.
Another point of reference for energy is the amount contained in one gallon of typical gasoline in your car. This is 120MJ (120x106 J). 120x106 J/3600s is 33.4x103 watts, or 33.4kwh. You could heat nearly 3800 cups of water to this tepid temperature with only one gallon of gasoline! Or you could drive 20 miles in a car. Or you could heat 3 baths with it (70 gallons of water per bath, water at 40°C, 16 cups in a gallon).
Alright, we are done discussing background energy for now. Onto the real stuff!
Lifecycle energy assessment of disposable extruded polystyrene (Styrofoam) cups vs. plastic and ceramic cups
In 1994, a professor named Hockins analyzed the energy use of disposable vs. ceramic cups. It includes the energy required to extract the components used to create the cups, the process used to form the compounds into cups, and for ceramic cups, the energy required to wash them.
Styrofoam cups (my word processor automatically capitalizes “Styrofoam” and I am too lazy to correct it) are a petroleum product called polystyrene. Styrofoam is the exact same material used in the disposable plastic silverware you use at picnics and other events, and also the cases that CDs come in. How does it get light and fluffy, then? In the manufacture of Styrofoam, gases are dissolved in the molten polystyrene under very high pressure. The Styrofoam is shaped into whatever shape they choose, then brought rapidly to low pressure (extruded, hence the name extruded polystyrene). The bubbles of gas dissolved in the polystyrene expand under low pressure, making Styrofoam mostly air (technically mostly a gas) with polystyrene holding it in place. They are typically disposed after one use. If disposed of properly, the polystyrene can end up at an incinerator where it produces mostly CO2 and H2O upon burning. In this way, part of the energy to make the cup can be reclaimed. If the Styrofoam is disposed of normally, it may be burnt to release black carbon and particulate matter, or end up in a landfill.
Ceramic cups are largely heated clay. The material needs to be mixed in the right proportions, shaped, and heated several times to dry and hold its form. The heating process requires a lot of energy. Moreover, washing the cups requires quite a bit of energy to heat the water for washing the cups.
Let’s get into the numbers.
Our reusable cups: A ceramic cup takes 14.1 MJ per cup to manufacture. A pyrex cup takes 5.5 MJ to manufacture, a reusable plastic cup takes 6.3 MJ. Our disposable cups: an uncoated paper cup is .5 MJ (500 kJ), and a Styrofoam cup is .2 MJ (200 kJ). The first thing we see is that manufacturing disposable cups requires between 4% (Styrofoam) and 10% (paper) of what it takes to make the most energy efficient reusable cups (glass and plastic). In other words, at very best, a plastic cup needs to be used 25 times to be as energy efficient as a Styrofoam cup, or 10 times to be as efficient as a paper cup. A ceramic coffee mug would require 75 uses before it would hit break-even energy as a Styrofoam cup, or 30 uses compared to a paper cup.
Now let’s account for the cost of washing. The same source indicates that a commercial washer requires between 80 and 120 kJ of energy to wash a single cup amongst an entire normal wash load. Compare this to the 31.5 kJ we calculated to heat coffee to a warm temperature. The differences arise in the amount of water necessary to wash and then rinse a cup in a commercial washer, and the higher temperature used in commercial washers compared to the temperature at which we drink in coffee or tea. A commercial washer has a wash cycle, a rinse cycle, and heats the water to a scalding 80 degrees C. Another important factor considered in this, which we will discuss in a later blog post, is the difference between energy used, and energy expended at a power plant to produce the energy used by the commercial washer. Power plants are not very efficient. The average power plant in the US will deliver only 35% of the energy from the fuel it burns. So we have to divide the wash energy by .35, getting a higher number of energy burnt to wash our cups.
Dividing the 80 kJ of energy of a dishwasher by a .35 efficiency results in nearly 230 kJ to wash a cup. This is more than it takes to produce a basic Styrofoam cup. In other words, in the US, no matter how many times you use a mug in a commercial setting, the total energy required will always exceed the energy used in a Styrofoam cup. Note that this is not the same as CO2 emissions (next post covers this!)
But hold on! Who uses a commercial dishwasher in their home? More importantly, who washes their cup every time they use it, besides my sister? Taking the former case and assuming we very efficiently wash the cups by hand, assuming it takes ½ a cup of warm water (heated up by 30°C) to wash a cup (both the temp and amount** of water use are highly optimistic!), and using ½ of what we calculated in the example section, it takes about 16kJ to wash a cup, or close to 45kJ accounting for the efficiency of electricity production. Now we are back to a break-even point closer to 100 uses for a ceramic mug, or around 35 for a glass! Not bad! And if you are like me, and you usually just rinse a mug with cold water and let it dry, or don’t wash it at all because you will be putting the same tea/water in next time, energy use is negligible, bringing us much closer to those 75 and 25 uses!
That’s enough for now. But there is so much left to discuss here! We need to consider many other factors. Pollution from production and disposal of disposable cups, energy sources used for electricity and how clean each is, recycling habits, landfill volume requirements etc.
What can we take away from this before we discuss all the other stuff? Be careful when you say that glasses and mugs are more energy efficient than Styrofoam or paper mugs. This takes many reuses. Another important implication: if you are throwing a party, you are better off buying paper cups (which can be recycled) and recycling them afterwards than you are buying glasses that will only be used during that party (or maybe one or two future parties).
This post is getting long, so I am going to sign off for now, and perhaps revisit this again later. Thanks for checking it out!
-Jason Munster
Edits (11-29):
* Lucas asked about a home dishwasher. A very efficient home dishwasher uses 3.6MJ of energy per wash (from EnergyStar). Assume you can cram 40 cups in there, you have 90kJ per cup, which would bring ceramic break-even re-uses to about 125, and glass to about 45.
** A lot of people are very wasteful when they wash dishes by hand, using a ton of hot water (as in running the water the entire time, probably using 8 cups of water to wash a cup). This is very inefficient and will never allow for break-even energy use.

Pingback: Coal Power Plants | Jason Munster's Energy and Environment Blog
Pingback: Climate Change 2 | Jason Munster's Energy and Environment Blog
Pingback: Disposable Paper Vs. Plastic Reusable Cups | Green Living Tips
You do have a good point here. Nice post!
Thank you for putting the science in non-engineer terms. This will help my 6th grade daughter grasp the complexity of the recycling issue that is at the core of her project on (wait for it!) disposable cups from convenience stores/fast food outlets.
Welcome! I am glad that young people are working on projects like this, and that my work writing can help.
Hello, i read your blog occasionally and i
own a similar one and i was just curious if you get a lot of spam remarks?
If so how do you prevent it, any plugin or anything
you can suggest? I get so much lately it's driving me insane so any assistance is
very much appreciated.
Thanks for your great post. It definitely helps with clarify the picture. At the office, we have paper, or can bring a mug from home, and I'm always debating which is better. This is what brought me to this post. One thing you didn't address is transportation energy. How much does it take to deliver one glass cup vs continuous delivery of paper. I've read that this component makes a big difference in the paper vs plastic bag debate, and was wondering if it makes a difference here.
(yeah, your post is somewhat old, but still relevant).
This is definitely true! This page here, despite being from a biased domain, gets the CO2 costs of shipping goods about right.
The article I just reference is in terms of grams of CO2. Most coffee mugs weigh around .35kg. Assuming it travels by freight in a truck for 1000km, it will produce all of 5 grams of CO2 to transport. Now my article was written in terms of energy. 360KJ of energy is roughly the grid-equivalent of 1000 grams of CO2. Given that a ceramic mug costs 14.1MJ to manufacture, it can take up to 50 kilograms of CO2 to produce one, depending on the power source. So that's 10,000 times larger than the manufacturing cost. In other words, for many consumer goods, the shipping emissions are tiny.
The weight of paper and styrofoam products, to a first order, scale with the lower energy costs to make them, so we'd get similarly low emissions for transportation. Maybe only 1000x as much emissions to produce as to transport, but it's still such a high multiple that transportation cost is not much of a concern.
I am a volunteer at my kids school and we are working on a project with 4th & 5th graders on Trash - Reduce, Reuse, Recycle. I am starting by having them research 6 different types of beverage containers - ceramic, glass, reusable plastic, paper, disposable plastic, and foam. It has been eye-opening for me as I put together materials to discuss with them. This type of post will be very helpful in our research.
You're welcome! There is a lot of bogus information out there. Unless it's backed up with basic physics and math, a lot of information is not trustworthy. Good luck!
Thanks for this post. Easy to read. I'll share it with my students. I am the department head of Geography at a high school in Kitchener, Ontario, Canada.
I learned much about the energy requirements of single-use vs glass or ceramic containers. You alluded to in the article that there would be another post about CO2 emissions, and I was wondering if that was available. Also, in any of your posts do you cover resource requirements in single-use vs. ceramic/glass containers?
I'm hoping to get a more clear picture of this. Many of my students buy drinks in throw-away containers and I'm considering banning them in my class. Any background information would be helpful.
Thanks again.
Hi! Sorry for the delay. The follow-on post can be found here. I don't mention much of resource requirements, since the inputs for most containers tend to be fairly simple. Did you end up banning throw-away containers in class?