This is my first post-startup-launch post. (If you haven't yet, check out my website and sign up for more information!)
I mentioned I wasn't going to post about the environment as much anymore, but I can't stop. I honestly started this just to show how fun math can be in looking at the number of views on Adele's Hello on youtube. It ended up with me being curious what the environmental impact was.
So today I looked at the youtube video of this song. For your convenience, here it is so you can listen to it while reading this post. Apparently it's blowing up, with an insane number of views per day.
It has 309,000,000 views and has been up for all of 20 days. Let's use some basic math to figure out how popular this song is!
Let's first see how many seconds there are in a day.
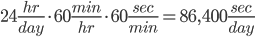
And over 20 days:
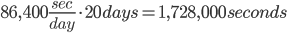
With 309,000,000 views in that time, we end up with 178 views initiated per second, on average, for 20 days, nonstop.
But, hold up, it takes 6 minutes for this song to complete. So that means, on average, there are:
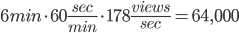
Let's round that up to 65,000. On average, at any given time, there are 65,000 people watching the Adele Hello video on youtube. There are 15 million views per day. That's just one video on one source. In eight whole day, as many people listen to the song, on just youtube, as watch the superbowl.
If this clip keeps up (which is unlikely), she will surpass Gangam Style, with over 2 billion views, in less than 150 days. And medical stocks will go up, cause I worry that listening to this song too frequently may drive people into depression.
"But Jay," you say, "what about the energy and environment component?!?"
Adele, impacting your heart, and impacting the environment
Let's figure out Adele's carbon footprint from youtube! Let's assume that Google's hosting emissions are negligible. Let's just focus on the energy used for a laptop. Let's say that it is 50W (this is conservative).
From a prior post, we know that a 100W lightbulb uses ~1kg of coal per day. So a laptop will use about 0.5kg per day. How much CO2 is this?
This is basic chem! Let's get really basic. Coal is mostly made up of carbon. So when it is burnt, every carbon molecule breaks off and bonds with O2. So we assume that all 0.5kg of carbon becomes CO2. You need to add that weight of the O2. C weighs 12 AMU (atomic mass units, pretty much the mass of a single molecule) and O2 weighs 32 AMU.
So we need to multiply the weight of coal, which is pure carbon, by the proportional increase of mass of CO2, cause each carbon atom gains O2 weight (12+32=44)
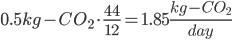 for a 50 watt computer running all the time.
for a 50 watt computer running all the time.
Also this song is 6 minutes, or 1/10th an hour, and an hour is 1/24th a day, so this song takes 1/240th of a day.
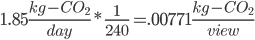
Don't forget, though, that we have 15,000,000 views per day!
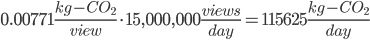
Or, you know, ~125 tons of CO2 per day. Just from Youtube and Adele. This is the generation rate of about 2000 Americans.
And why this analysis overestimates Youtube's and Adele's contribution to CO2 emissions
Most people are multi-tasking while listening to Adele (ie those 50 watts they are using are also going towards whatever else they do while listening to Adele, such as reaching for tissues to blot their tears), so you have to take a fraction of this number 🙂
Second, much of the power use in the US is now coming from natural gas, which is more efficient than coal, so you can cut down this number.
Third, tissues take a whole lot of energy to make. I'm betting that the raw number of tissues used while listening to Adele will increase the CO2 footprint.
How many times did you restart the song while reading this blog, BTW?
Thanks for reading!
- Jason Munster
